Which of the Following Best Describes an Arrhenius Acid-base Reaction
It is limited to situations that involve aqueous solutions or specific compounds. Hence Arrhenius acid-base reaction is the combination of an acid and base to produce salt and water as products.

Which Of The Following Best Describes An Arrhenius Acid Base Reaction Brainly Com
Which of the following best describes an Arrhenius acid-base reaction.
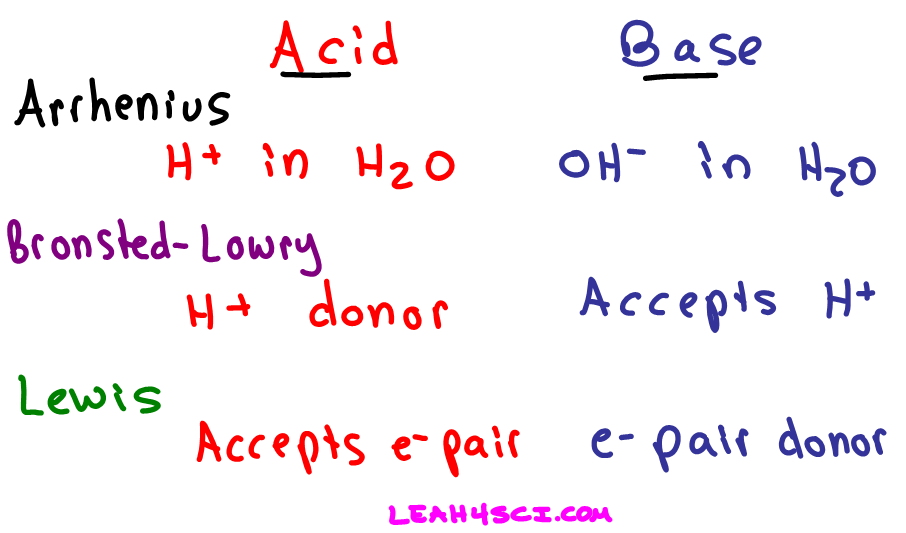
. Acid base salt water B. It absorbs hydrogen ions in solution C. Which of the following statements best describes the behavior of water in the acid-base reaction between dopamine and water.
Acid base H. Acid base conjugate base conjugate acid B. Hydronium breaks up to yield an H in solution.
According to the Arrhenius description of acids and bases the water molecule consists of a proton and a hydroxide ion. Acid base H20 B. Water is the conjugate base.
It donates a proton. Which statement describes the Arrhenius interpretation of acids and bases. Acid base H OH-C.
Water is the conjugate acid. Acid base H20 B. Water is both an acid and a base.
It produces water in a chemical reaction b. 3 on a question Which of the following best describes an Arrhenius acid-base reaction. It produces OH in solution.
Acid base salt water. An Arrhenius acidbase reaction occurs when a dissolved aqueous acid and a dissolved aqueous base are mixed together. HO H0 oth HO ног HO H20 NH2 dopamine a.
Hydroxide is an OH- dissolved in water. - 16155652 kyleroberts610oy97pv kyleroberts610oy97pv 05042020 Chemistry Middle School answered Which of the following correctly describes an Arrhenius acid. Previous Next.
Acid base H OH- O C. Acid base salt water. An Arrhenius base is a substance that when added to water increases the concentration of OH 1-ions present.
It releases hydroxide ions in solution It releases hydrogen ions in solution d. Arrhenius Acid Definition. Which of the following describes a reaction that reaches equilibrium.
The acid-base reaction is considered a type of neutralization reaction where the acid and base react to yield water and a salt. Hydronium is an H donor regardless of solution. Acid base salt water.
This is a neutralization reaction. Correct answer - Question 21 of 25 Which of the following best describes an Arrhenius acid-base reaction. 1 question Asap classify the following reaction as arrhenius brønsted-lowry or lewis acid-base.
HCLO3 NH3 - NH4 ClO3-. HCl is an example of an Arrhenius acid and NaOH is an example of an Arrhenius base. Which of the following best describes an Arrhenius acid-base reaction.
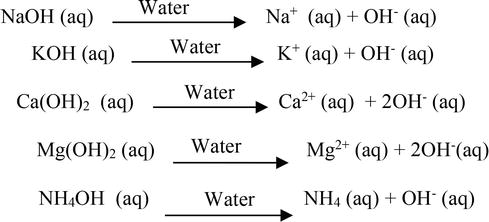
NaOH aq Na aq OH aq Some other examples of Arrhenius base are 1st and 2nd group. Water is the acid. Water is the base.
A compound is an Arrhenius acid if. Hence Arrhenius acid-base reaction is the combination of an acid and base to produce salt and water as products. Acid base H OH O D.
The reaction may fit all two one or none of the categories. Acid base salt water. The chemical formulas of Arrhenius acids are written with the acidic hydrogens first.
The product of such a reaction is usually said to be a salt plus water and the reaction is often called a neutralization reaction. Acid base H OH- C Acid base salt water D. Acid base H2O D.
3 on a question Which of the following best describes an Arrhenius acid-base reaction. A general definition based on electron structure. The H 1 ion produced by an Arrhenius acid is always associated with.
First one is the correct answer. Which of these statements best describes the use of an acid-base indicator. The products reach a maximum level and then begin to drop off.
It accepts a lone pair of electrons. Acid base H OH- B. Which is a base-conjugate acid pair.
Which of the following correctly describes an Arrhenius acid. Hydroxide attacks and accepts the H from hydronium. Test your knowledge on arrhenius acid.
As a result the interaction between an acid and a base in Arrhenius acidbase reactions is a neutralization reaction. Narendra modi is currently the pm of india. A substance that indicates the pH of a solution b.
Acid base conjugate base conjugate acid. Acid base conjugate base conjugate acid O C. Question 7 of 25 Which of the following best describes an Arrhenius acid-base reaction.
In an aqueous solution an Arrhenius acid raises the concentration of hydrogen H ions while an Arrhenius base raises the concentration of hydroxide OH ions. Consider the reaction below. Acid base H20 O B.
Acidity and alkalinity describe the concentration of hydrogen ions acidity and hydroxide ions alkalinity. Acid base. What equation best describes an Arrhenius acid base reaction.
The Arrhenius Acid And Base Theory Intechopen
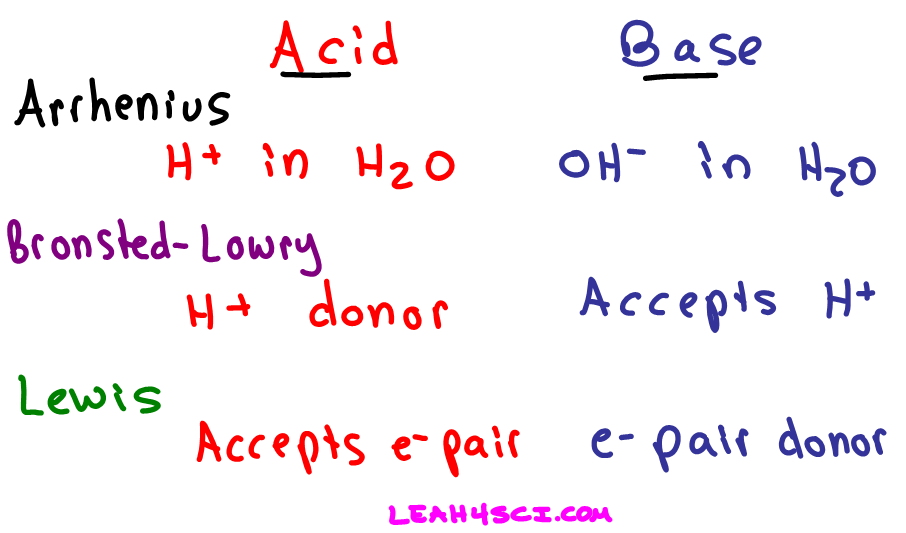
Definitions Of Arrhenius Bronsted Lowry And Lewis Acids And Bases In Organic Chemistry

𝙿𝚒𝚗𝚝𝚎𝚛𝚎𝚜𝚝 𝚕𝚞𝚑𝚑𝚋𝚡𝚡𝚋𝚢𝚢𝚢𝚢 Video Science Notes Physics Notes School Organization Notes
No comments for "Which of the Following Best Describes an Arrhenius Acid-base Reaction"
Post a Comment